Guided Specific Heat Lab
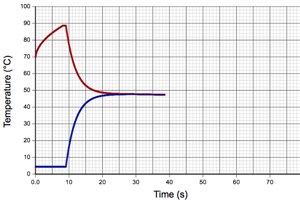
In this guided lab you will determine the specific heat of an unknown liquid. You will mix about 100 mL of hot water with 100 mL of a unknown liquid and monitor the change in temperature to each liquid. You will then set the heat lost by the hot water equal to the heat gained by the unknown. This will allow you to find the specific heat of the unknown.
When you are ready to start this activity, click on the begin button. When finished with the online version, you should redo the steps with real equipment.
When you are ready to start this activity, click on the begin button. When finished with the online version, you should redo the steps with real equipment.
Begin
Don't put units in the submission box.
Specific Heat (J/(kg*°C)):
Your Full Name:
Submit
Recollect
Scale Lab Completion Certificate