Heat Transfer Problem With Entropy
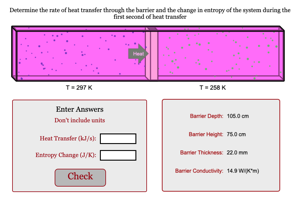
In this problem you will have to determine initial rate of heat transfer through a barrier that is separating two gases at different temperatures and then you will use this rate to determine the change in entropy of the system during the first second of heat transferThe container itself is insulated so that no energy is exchanged between the gases and the surroundings of the system. Their will be negligible change in the temperature of either gas during this first second of heat transferClick begin to work on this problem
Name:
Begin
See Available Languages
Determine the rate of heat transfer through the barrier and the change in entropy of the system during the first second of heat transfer
Enter Answers
Don't include units
Heat Transfer (kJ/s):
Entropy Change (J/K):
Check